
Examining the Clinical Utility of PrismRA
PrismRA® is a blood-based precision medicine test used by over 1,400 advanced practitioners around the country to guide the treatment selection in their patients with rheumatoid arthritis.
While validated precision medicine testing has grown in other disease areas such as cancer, prior to PrismRA predictive biomarkers used to inform RA treatment selection had not been validated or replicated, thus limiting their clinical utility.2-4 As a result, rheumatologists must often take a trial-and-error treatment approach, which does not tailor therapies to a patient’s individual disease biology and can lead to poor patient outcomes such as more surgeries or visits to the emergency room.1
PrismRA is the first of its kind precision medicine test that has been clinically validated to look at predictive biomarkers to inform RA treatment selection.
The clinical utility of PrismRA to get patients on the right therapy sooner was evaluated across multiple, real-world, prospective studies.
Clinical Utility First Interim Analysis
Published in the January 2022 issue of Expert Review of Molecular Diagnostics journal the study reports an interim analysis from the AIMS study, which is a large scale, prospective study to collect longitudinal patient clinical and molecular data to evaluate clinical utility.2 Data was collected at baseline, treatment initiation, and 12- and 24-week follow-up visits. The primary endpoint of the outcome analysis was therapeutic responsiveness defined by achievement of ACR50 at 24 weeks.

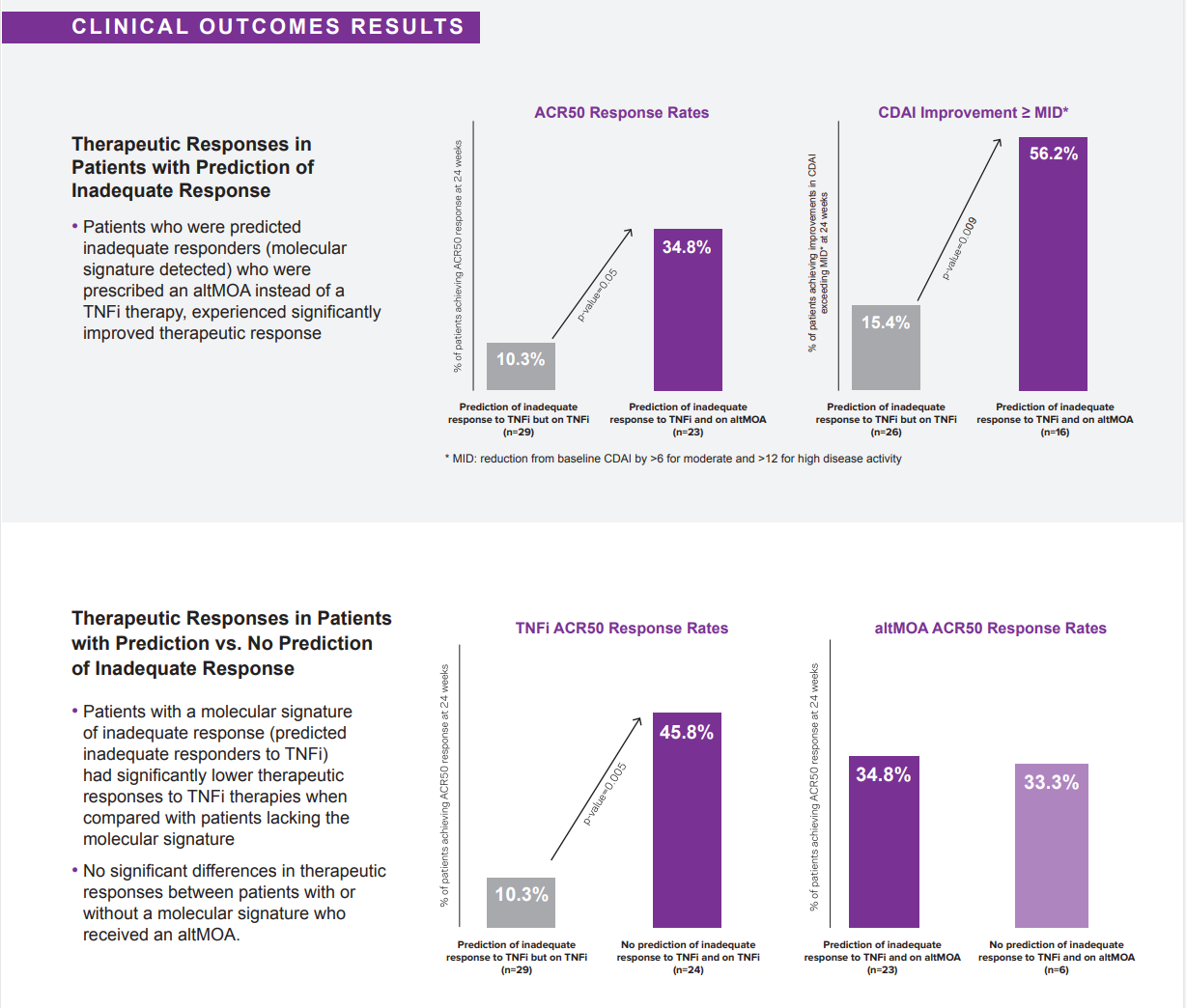
Results of the study show patients who were predicted inadequate responders to TNFi therapies who were prescribed a therapy with an alternative mechanism of action (altMOA) instead of a TNFi therapy, experienced over 3x improvement in therapeutic response according to both ACR50 at 6 months and CDAI minimally important difference (MID).2
Clinical Utility Second Interim Analysis
The second prospective study on the clinical utility of PrismRA was published in June 2022 and looked at the absolute change in CDAI scores depending on both the PrismRA result and the therapy selected. Results of this study showed when therapy selection was guided by the PrismRA test patients experienced significant improvement in disease activity.3

*PrismRA result of inadequate response detected is categorized as either 1) patient has a 10% chance of responding to TNFi or 2) patient has a 5% chance of responding to TNFi. A low score does not ensure a positive response to TNFi therapies.
Patients with a prediction of inadequate response to TNFi who initiated a therapy with an altMOA had a near 2x improvement in their CDAI score (14.2) at 6 months, compared to patients with the same prediction of inadequate response who initiated a TNFi (7.8).3
Patients without a prediction of inadequate response who initiated a TNFi experienced a 1.6x improvement in CDAI scores at 6 months (12.7), compared to patients who had a prediction of inadequate response to TNFi therapies (7.8).3
Clinical Utility Third Interim Analysis
Published in November of 2022, this clinical utility study on PrismRA show that patients were 2-3 times more likely to reach treatment goals, such as low disease activity or remission, when PrismRA guided their therapy selection compared to current care.4


Selecting the right target therapy after a patient with RA fails methotrexate can be challenging. PrismRA helps rheumatologists identify patients who are likely to fail TNFi therapies and start them on therapies better suited for their biology sooner.
Click here to read a clinical summary of the PrismRA clinical utility first interim analysis, Strand et al.
Click here to read a clinical summary of the PrismRA clinical utility second interim analysis, Strand et al.
Click here to read a clinical summary of the PrismRA clinical utility third interim analysis, Curtis et al.
1. Grabner M, Boytsov NN, Huang Q, et al. Costs associated with failure to respond to treatment among patients with rheumatoid arthritis initiating TNFi therapy: aretrospective claims analysis. Arthritis Res Ther. 2017;19(1):92. Published 2017 May 15. doi:10.1186/s13075-017-1293-1. 2. Strand V, Cohen SB, Curtis JR, et al. Clinical utility of therapy selection informed by predicted nonresponse to tumor necrosis factor-α inhibitors: an analysis from the Study to Accelerate Information of Molecular Signatures (AIMS) in rheumatoid arthritis. Expert Rev Mol Diagn. 2022 Jan;22(1):101-109. doi: 10.1080/14737159.2022.2020648. 3. Strand V, Zhang L, Arnaud A, et al. Improvement in clinical disease activity index when treatment selection is informed by the tumor necrosis factor-a inhibitor molecular signature response classifier: analysis from the study to accelerate information of molecular signatures in rheumatoid arthritis. Expert Opin Biol Ther. 2022 Jun;22(6):801-807. doi: 10.1080/14712598.2022.2066972. 4. Curtis JR, Strand V, Golombek S, et al. Patient outcomes improve when a molecular signature test guides treatment decision-making in rheumatoid arthritis. Expert Rev Mol Diagn. 2022 Nov;22(10):973-982. doi: 10.1080/14737159.2022.2140586.
